Gel Filtration Chromatography Media
Gel filtration chromatography is also called exclusion chromatography or molecular sieve method, which is mainly based on the size and shape of the protein, that is, the weight of the protein for separation and purification. The packing materials in the chromatography column are some inert porous network structure substances, mostly cross-linked glycans (such as dextran or agarose) so that protein mixtures could be separated according to different molecular sizes. Generally, large molecules flow out first and small molecules flow out later.
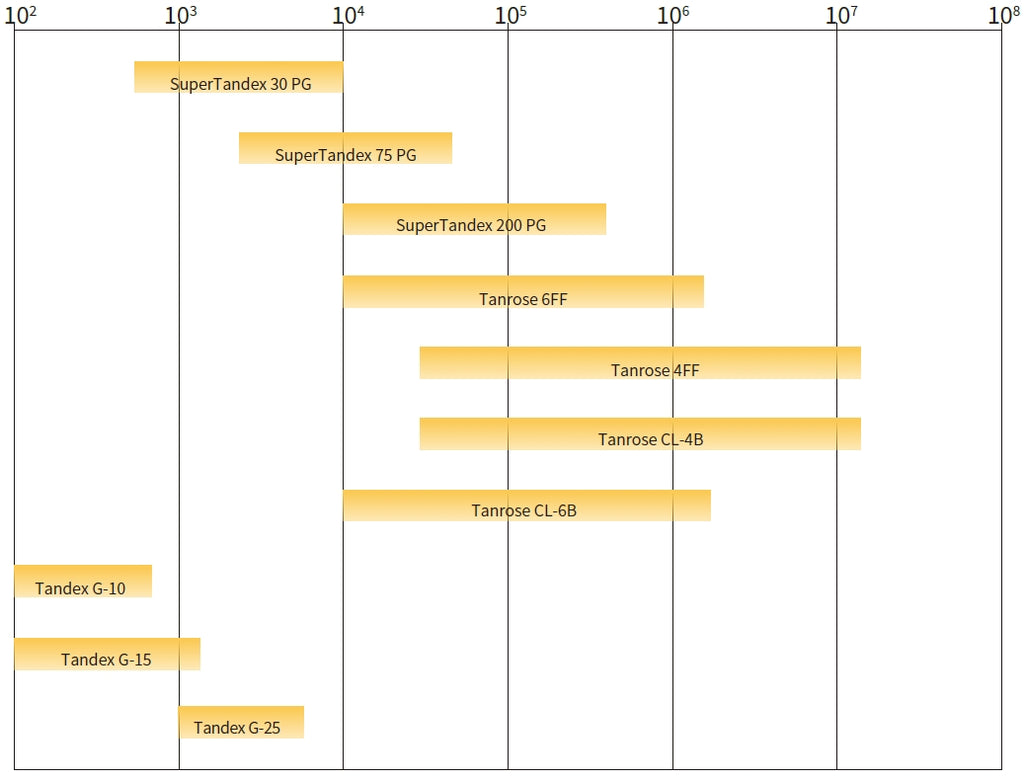
Therefore, the key to selecting gel filtration media is to choose a suitable separation range, and then mechanical properties and scalability of the media can be taken into account.
Advantages
- No charge, weak adsorption, mild operating conditions
- Can work in a wide temperature range
- No need of organic solvents
Selection of Gel Filtration Chromatography Columns
For group separation, gel filtration chromatography columns with a length of 2-30 cm are generally used.
For gradient separation, columns with a length of around 100 cm and a diameter in the range of 1-5 cm are usually required. A diameter smaller than 1 cm produces wall effects, while a diameter larger than 5 cm results in significant dilution. The length-to-diameter ratio (L/D) is generally recommended to be between 7-10, but for substances with slow mobility, it should be between 30-40.
Gel filtration separating list (Da, protein globules)
Ion-Exchange Chromatography Media
The separation of proteins by ion exchange chromatography is carried out according to the different charges of proteins under a certain pH condition. Due to most biological molecules have acidic or alkaline groups, anion exchange media can bind with the negatively charged proteins whilst cation exchange media can bind with the positively charged proteins. Through adjusting the pH of buffer, proteins with poor binding capacity will be eluted first, proteins with strong binding capacity will be eluted later.
Advantages
- Good maneuverability
- Fast flow rate, high productivity
- Moderate bead, good resolution
- Good physical and chemical stability, suitable for the initial capture or moderate purification of various sizes of biomolecules
- High purification technique, can be used in combination with hydrophobic chromatography
Classification and features

Hydrophobic Chromatography Media
Hydrophobic chromatography is a method to separate biological macromolecules according to their surface hydrophobicity. Some hydrophobic groups are often exposed to the surface of biological macromolecules (such as proteins and peptides). Hydrophobic groups can bind with hydrophobic chromatography media by hydrophobic interaction. Due to the different hydrophobicity of various molecules, the hydrophobic effect between molecules and media is different. Hydrophobic chromatography separates and purifies biological macromolecules according to this principle.
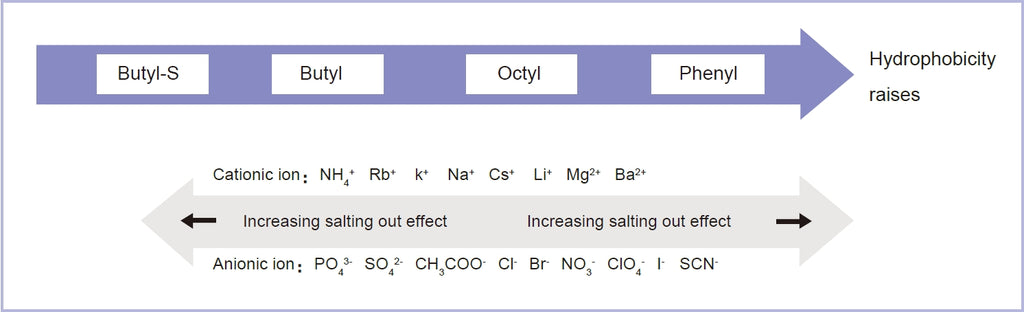
The key to choosing the hydrophobic chromatography medium is to select ligand with appropriate hydrophobic effect. Proteins with
strong hydrophobicity need to match medium with weak hydrophobicity, and vice versa. In order to enhance the combination of protein and hydrophobic medium, a certain amount of salts (usually ammonium sulfate) need to be added in the buffer. If protein itself has strong hydrophobicity, there is no need to add too much salt. For the purpose of improving the resolution, the hydrophobic medium with
smaller beads can be selected.
Factors influencing hydrophobic chromatography
- The hydrophobicity of a protein depends on the distribution of hydrophobic groups on its surface.
- Some ions in the buffer contribute stability to the conformation of proteins. For example, SO4 2- can improve the stability of protein structure, reduce the solubility of proteins, have salting-out effect on proteins, and enhance the hydrophobic effect between proteins and ligands. Some ions contribute instability to the conformation of proteins, for instance, Cl- , Ca2+ can increase the solubility of protein, and these ions usually have strong elution ability. The characteristics of salting out and salt solution can be used as the basis for choosing the equilibrium and elution conditions of hydrophobic media.
- In the process of hydrophobic chromatography, the higher the temperature, the stronger the hydrophobic effect, which helps improve separation degree of chromatography columns. However, for bioactive substances, high temperature will lead to denaturation and inactivation. Therefore, it is recommended to keep room temperature or low temperature during the process of hydrophobic chromatography.
- Neutral phosphate buffer is usually used as the mobile phase of hydrophobic chromatography. With the increase of pH, the interaction between proteins and hydrophobic groups will decrease, because charge of acidic groups of proteins and hydrophilicity of proteins increases as pH increases. However, method that changing the pH of the solution is rarely adopted to change hydrophobicity of proteins in hydrophobic chromatography.
Affinity Chromatography Media
Affinity chromatography is a method to separate biomolecules based on the characteristics of specific recognition and reversible binding between biomolecules and some corresponding specific molecules. Affinity chromatography is an very effective method to separate proteins, which usually takes one step to obtain proteins of high purity. Proteins are separated according to their specificity to specific ligands rather than their covalent binding capacity.
Features
- Efficient, fast and convenient
- Strong selectivity
- Usually take one step to obtain proteins of high purity
- High recovery rate
Composition of Affinity Medium
Structure: agarose, such as Tanrose FF, Solid Mustang, etc.
Ligand: a substance that interacts specifically with a target molecule.
Brand: PreCot
Separation Mode: Ion Exchange
Base material: Agarose
Bead Structure: 6FF
Founctional Group: SP
Specification: 5mL
Gel Filtration Chromatography Media
Gel filtration chromatography is also called exclusion chromatography or molecular sieve method, which is mainly based on the size and shape of the protein, that is, the weight of the protein for separation and purification. The packing materials in the chromatography column are some inert porous network structure substances, mostly cross-linked glycans (such as dextran or agarose) so that protein mixtures could be separated according to different molecular sizes. Generally, large molecules flow out first and small molecules flow out later.
Therefore, the key to selecting gel filtration media is to choose a suitable separation range, and then mechanical properties and scalability of the media can be taken into account.
Advantages
- No charge, weak adsorption, mild operating conditions
- Can work in a wide temperature range
- No need of organic solvents
Selection of Gel Filtration Chromatography Columns
For group separation, gel filtration chromatography columns with a length of 2-30 cm are generally used.
For gradient separation, columns with a length of around 100 cm and a diameter in the range of 1-5 cm are usually required. A diameter smaller than 1 cm produces wall effects, while a diameter larger than 5 cm results in significant dilution. The length-to-diameter ratio (L/D) is generally recommended to be between 7-10, but for substances with slow mobility, it should be between 30-40.
Gel filtration separating list (Da, protein globules)

Ion-Exchange Chromatography Media
The separation of proteins by ion exchange chromatography is carried out according to the different charges of proteins under a certain pH condition. Due to most biological molecules have acidic or alkaline groups, anion exchange media can bind with the negatively charged proteins whilst cation exchange media can bind with the positively charged proteins. Through adjusting the pH of buffer, proteins with poor binding capacity will be eluted first, proteins with strong binding capacity will be eluted later.
Advantages
- Good maneuverability
- Fast flow rate, high productivity
- Moderate bead, good resolution
- Good physical and chemical stability, suitable for the initial capture or moderate purification of various sizes of biomolecules
- High purification technique, can be used in combination with hydrophobic chromatography
Classification and features

Hydrophobic Chromatography Media
Hydrophobic chromatography is a method to separate biological macromolecules according to their surface hydrophobicity. Some hydrophobic groups are often exposed to the surface of biological macromolecules (such as proteins and peptides). Hydrophobic groups can bind with hydrophobic chromatography media by hydrophobic interaction. Due to the different hydrophobicity of various molecules, the hydrophobic effect between molecules and media is different. Hydrophobic chromatography separates and purifies biological macromolecules according to this principle.
The key to choosing the hydrophobic chromatography medium is to select ligand with appropriate hydrophobic effect. Proteins with
strong hydrophobicity need to match medium with weak hydrophobicity, and vice versa. In order to enhance the combination of protein and hydrophobic medium, a certain amount of salts (usually ammonium sulfate) need to be added in the buffer. If protein itself has strong hydrophobicity, there is no need to add too much salt. For the purpose of improving the resolution, the hydrophobic medium with
smaller beads can be selected.
Factors influencing hydrophobic chromatography
- The hydrophobicity of a protein depends on the distribution of hydrophobic groups on its surface.
- Some ions in the buffer contribute stability to the conformation of proteins. For example, SO4 2- can improve the stability of protein structure, reduce the solubility of proteins, have salting-out effect on proteins, and enhance the hydrophobic effect between proteins and ligands. Some ions contribute instability to the conformation of proteins, for instance, Cl- , Ca2+ can increase the solubility of protein, and these ions usually have strong elution ability. The characteristics of salting out and salt solution can be used as the basis for choosing the equilibrium and elution conditions of hydrophobic media.

- In the process of hydrophobic chromatography, the higher the temperature, the stronger the hydrophobic effect, which helps improve separation degree of chromatography columns. However, for bioactive substances, high temperature will lead to denaturation and inactivation. Therefore, it is recommended to keep room temperature or low temperature during the process of hydrophobic chromatography.
- Neutral phosphate buffer is usually used as the mobile phase of hydrophobic chromatography. With the increase of pH, the interaction between proteins and hydrophobic groups will decrease, because charge of acidic groups of proteins and hydrophilicity of proteins increases as pH increases. However, method that changing the pH of the solution is rarely adopted to change hydrophobicity of proteins in hydrophobic chromatography.
Affinity Chromatography Media
Affinity chromatography is a method to separate biomolecules based on the characteristics of specific recognition and reversible binding between biomolecules and some corresponding specific molecules. Affinity chromatography is an very effective method to separate proteins, which usually takes one step to obtain proteins of high purity. Proteins are separated according to their specificity to specific ligands rather than their covalent binding capacity.
Features
- Efficient, fast and convenient
- Strong selectivity
- Usually take one step to obtain proteins of high purity
- High recovery rate
Composition of Affinity Medium
Structure: agarose, such as Tanrose FF, Solid Mustang, etc.
Ligand: a substance that interacts specifically with a target molecule.
Brand: PreCot
Separation Mode: Ion Exchange
Base material: Agarose
Bead Structure: 6FF
Founctional Group: SP
Specification: 5mL

