Test Report
Chromatography conditions:
Column |
Welch Ultisil®XB- C18(4.6*250mm*5μm,300A) |
|||||||||||||||
Mobile Phase |
Phase A: Sodium Chloride Phosphate Buffer: Acetonitrile (75:25) Phase B: Sodium Chloride Phosphate Buffer: Acetonitrile (35:65) |
|||||||||||||||
Column Temperature |
35℃ |
|||||||||||||||
Detector |
UV |
|||||||||||||||
Injection Volume |
20μL |
|||||||||||||||
Flow Rate |
1.0 ml/min |
|||||||||||||||
Detect wavelength |
214 nm |
|||||||||||||||
Elution procedure |
|
|||||||||||||||
Note |
/ |
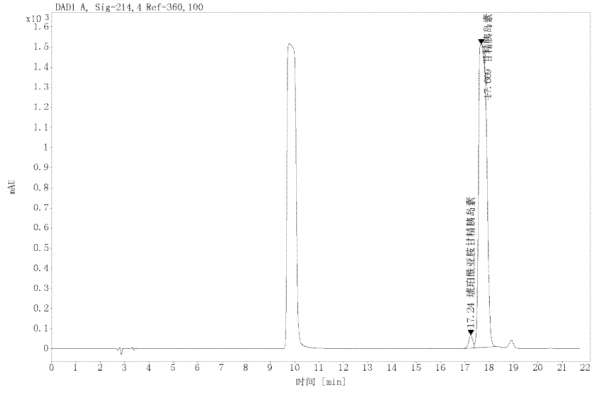
Chromatogram and Data:

Peak name |
Retention time (min) |
Peak Area |
Peak area(%) |
Resolution(USP) |
Tailing factor |
Number of plates(USP) |
peak to valley ratio |
Succinimide Insulin glargine |
17.24 |
724.58 |
2.029 |
|
0.88 |
51299.8 |
3.356 |
Insulin glargine |
17.67 |
34980.08 |
97.97 |
1.05 |
1.50 |
19096.8 |
78.909 |
Conclusion:
Welch HPLC column Ultisil XB-SAX (4.6×250mm, 5μm) was used to analyze the test solution under these chromatographic conditions. The retention time of the main substance was 26.1min and the chromatographic peak separation with adjacent substances was 1.71, which met the analysis requirements.