Distinction of Related Concepts About Liquid Chromatography
High performance liquid chromatography (HPLC) is a fundamental analytical discipline with many similar concepts. Therefore, it’s necessary for us to learn how to distinguish some easily-confused concepts of liquid chromatography.
1. Water Peak & Negative Peak
In liquid chromatography, the water peak usually appears as a solvent peak, so it is usually located in the solvent peak position (between 0 and 5min) for the appearance time. Compared with the solvent peaks of other organic solvents, the water peak commonly manifests as obvious baseline fluctuation and even negative peaks.

However, the negative peak in chromatographic analysis is not limited to the water solvent peak, so the negative peak may appear anywhere in the chromatographic analysis. In this case, the reason for the negative peak usually is that there are impurities causing negative absorption through the detector at this wavelength, or even the detector electrode is connected in the opposite direction.

2. Solvent Peak & Blank Peak
Solvent peak, as the name implies is the peak caused by dissolving sample solvent. If the sample is dissolved in methanol, the peak which appears when methanol solvent is injected is the solvent peak.
The concepts of blank peak and solvent peak are often confused by analysts. But strictly speaking, blank peak refers to the appearance condition of the sample matrix background (i.e., the blank matrix sample), not just the solvent peak. For simple small molecule polar compounds, there is usually no significant difference between solvent peak and blank peak on the spectrum. However, for protein, food and other samples that cannot ignore the matrix background interference, the blank peak is often more complex than the solvent peak.
3. Run with Blank Solvent & Runs with Empty
In the process of finding reasons for abnormal chromatographic peak, we often need to find the source of abnormal peaks, which often requires two steps:
Step 1. Runs with empty
Step 2. Runs with blank solvent
The concepts of these two steps must not be confused. Running with blank solvent refers to its literal meaning. Running with empty, does not mean to run with a blank solvent, also does not mean to inject by taking off the column and connect with a 2-way valve, but means to let the instrument run once without anything. If abnormal peak appears in the process of chromatographic operation, it is suggested to check according to the order illustrated above. If the results of step1 and 2 are normal, it indicates that the abnormal peak comes from the sample itself (matrix background). If step 1 is normal while step 2 is not, indicating that the abnormal peak comes from the blank solvent. If step 1 is abnormal, it indicates that the abnormal peak is caused by system contamination (including instrument, pipeline, injection system, mobile phase), or chromatographic column contamination.
4. Ghost Peak & Interference Peak
Ghost peak: a general term for chromatographic peaks of unknown origin. In the process of chromatographic separation, especially in the condition of gradient elution or too long using time of instrument, it is easy to see ambiguous chromatographic peak. Therefore, the most obvious characteristic of ghost peak is "erratic" and "spooky", this characteristic is usually reflected in the unstable retention time and peak area. Ghost peaks can ascribe to many aspects, but ghost peaks caused by gradient change of mobile phase is the trickiest and most common:
| Possible reasons | Explanation | Solution | |
| Pollution | Injection valve pollution | The injected sample contaminated the injection valve, such as the quantitative loop | When analyzing complex samples, clean the injection valve after each analysis |
| Flow cell pollution | The flow cell adsorbs certain compounds, which are gradually eluted during analysis | Clean the detector regularly | |
| Flow way pollution | The injection volume is too large, or there is pollution in the flow way caused by the previous project, which is eluted under the existing elution system | Clean the instrument with 100ml 40 ℃ water-water at room temperature-methanol-isopropanol- methanol | |
| Filter material pollution | Microorganisms grow on the filter, or substances dissolve out of the filter material | Replace the material with more reliable filtering consumables | |
| Solvent pollution | Sample solvents or mobile phase reagents are contaminated. Under the gradient condition, with the increase of the elution intensity of mobile phase, the pollutant is gradually eluted | Choose high purity solvents and reagents. Select low absorption additives. Do not use long stored solvents. Use the Ghost-Buster column | |
| Mobile phase metamor-phism | The mobile phase, especially the aqueous/salt phase, has not been replaced for more than 24 hours cause metamorphism | Change mobile phase frequently | |
| Residue | Injection residue | The sample is overloaded and residual in the injector, flow way, or column | Timely injecting and cleaning |
| System | Gradient change | In the ultraviolet detector, with the change of proportion of the two terms, the baseline will changed to some extent. When the change rate is large, gradient peaks will easily appear | Try to avoid the gradient change rate is too large. Use the Ghost-Buster column. |
| Blind side of flow way | For example, T - type fitting of pressure gauge, 90° turning angle used to connect flow way, etc | Try to avoid bending flow way |
Since the interference peak is known to be derived from pollutants, the biggest difference between the interference peak and ghost peak is that the peak time is often fixed (sometimes the peak area does not change too much). Therefore, confronting with interference peak, we can find reasons in aspects of the chromatographic column pollution, sample pollution, reagent pollution, mobile phase pollution.
5. Repeatability & Reproducibility
Repeatability and reproducibility are used to evaluate the precision of the analytical results, both show as the RSD of analytical results, but the actual meaning is not the same.
Repeatability: refers to the precision of analytical results obtained by the same analyst under the same experimental environment.
Reproducibility: refers to the precision of analytical results obtained by different analysts or different laboratories under the same experimental conditions in their respective experimental environments.
Compared with repeatability, reproducibility requires higher precision. Good repeatability doesn’t mean good reproducibility. But if reproducibility is good, repeatability must be good. For modern instrumental analysis, repeatability is easy to achieve. Reproducibility is of greater practical significance, which is also regarded as the condition that must be investigated by method verification.
6. Column Bed Collapse & Phase Collapse
Column bed collapse is also called packing material collapse, which refers to the visible gap appears in column entrance after column used for a period of time. The existence of the gap increases the dead volume, which leads to the decrease of column efficiency. The reasons for column bed collapse are as follows:
Reason 1: Pressure is too low when column is packing. Gaps begin to appear after column has been used for a period of time under high pressure.
Reason 2: That the operating pressure exceeds the max. pressure causes packing particles break up to create gap.
Reason 3: Extreme mobile phase (such as pH>8.0), dissolving packing materials leads to gap.
Reason 4: Extreme mobile phase (such as pH<1.5), causing the bonding phase fall off and the packing materials become loose.
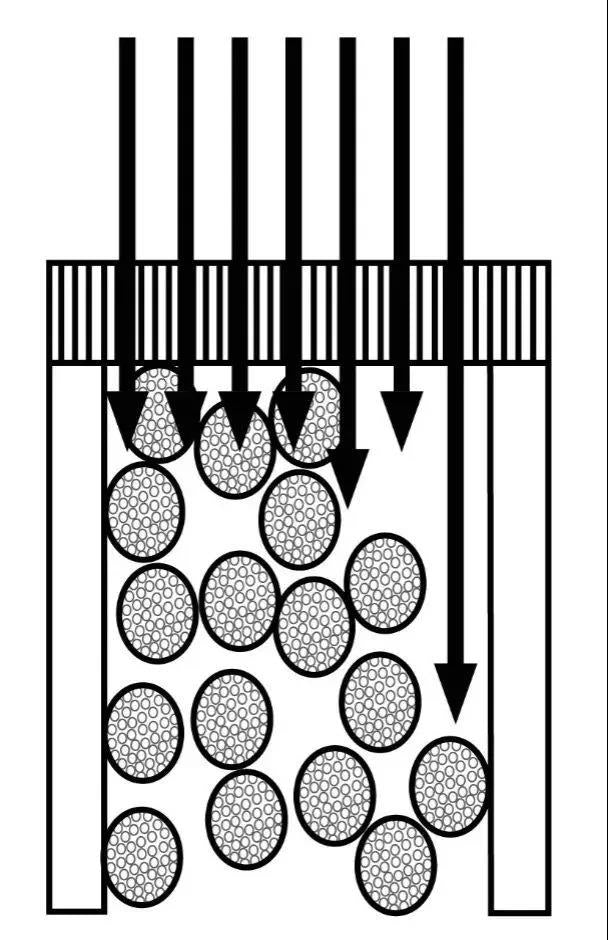
Bond phase collapse refers to that the bonded phase fails to stretch fully due to the discrepancy between the polarity of the mobile phase and the bonded phase is considerable. For instance, common C18, whose phase collapse can cause that column has insufficient retention to compounds under the condition of high proportion of water phase. Simple phase collapse does not lead to damage of column bed packing materials and is usually recoverable.

(a) carbon chain shape under methanol water mobile phase

(b) carbon chain shape under 100% water mobile phase
